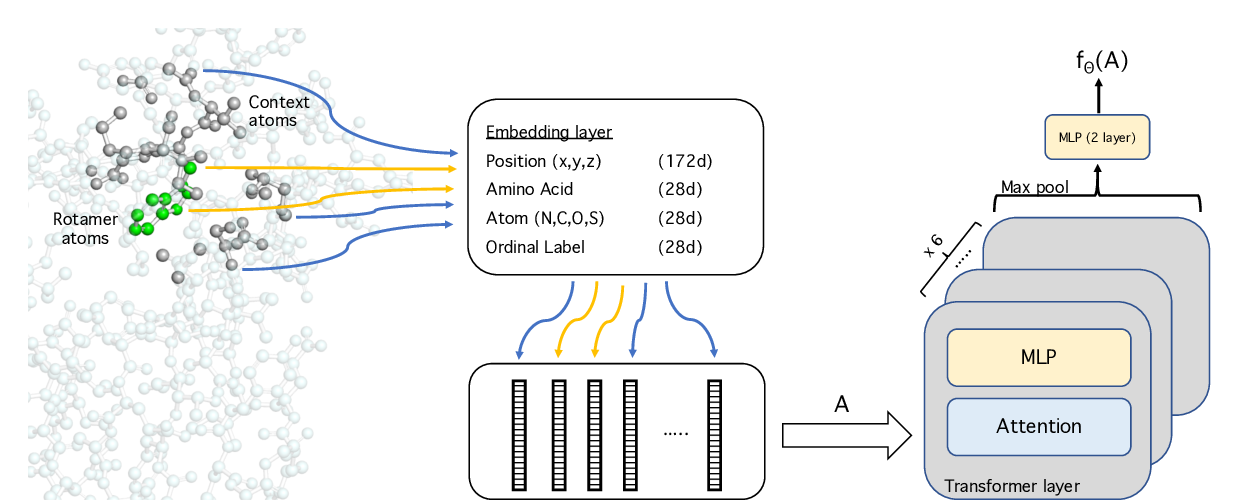
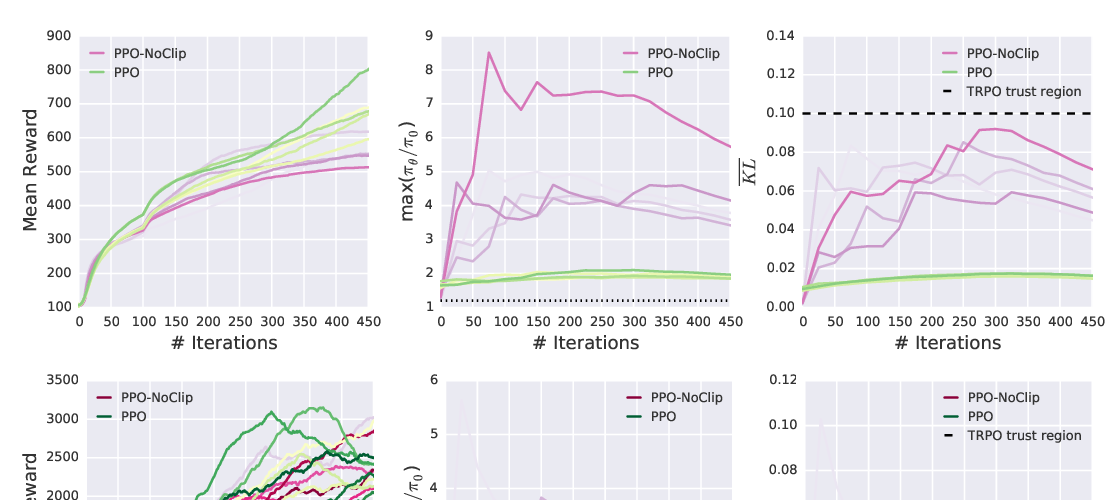
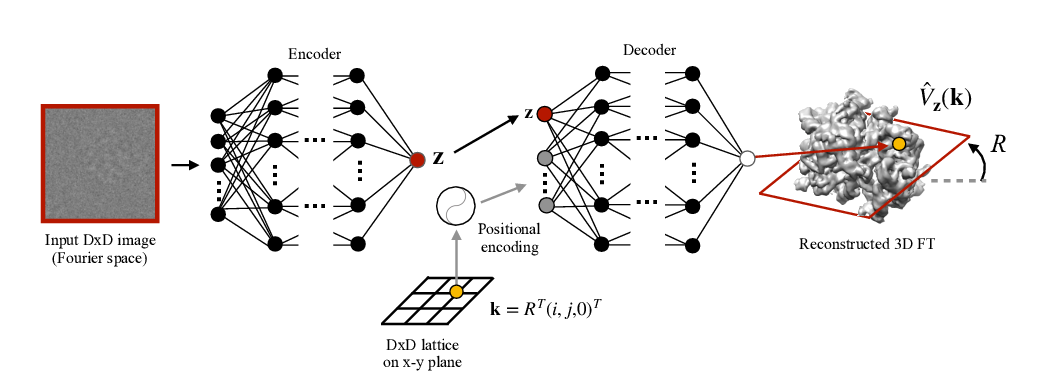
Abstract:
The ability to design biological structures such as DNA or proteins would have considerable medical and industrial impact. Doing so presents a challenging black-box optimization problem characterized by the large-batch, low round setting due to the need for labor-intensive wet lab evaluations. In response, we propose using reinforcement learning (RL) based on proximal-policy optimization (PPO) for biological sequence design. RL provides a flexible framework for optimization generative sequence models to achieve specific criteria, such as diversity among the high-quality sequences discovered. We propose a model-based variant of PPO, DyNA-PPO, to improve sample efficiency, where the policy for a new round is trained offline using a simulator fit on functional measurements from prior rounds. To accommodate the growing number of observations across rounds, the simulator model is automatically selected at each round from a pool of diverse models of varying capacity. On the tasks of designing DNA transcription factor binding sites, designing antimicrobial proteins, and optimizing the energy of Ising models based on protein structure, we find that DyNA-PPO performs significantly better than existing methods in settings in which modeling is feasible, while still not performing worse in situations in which a reliable model cannot be learned.